


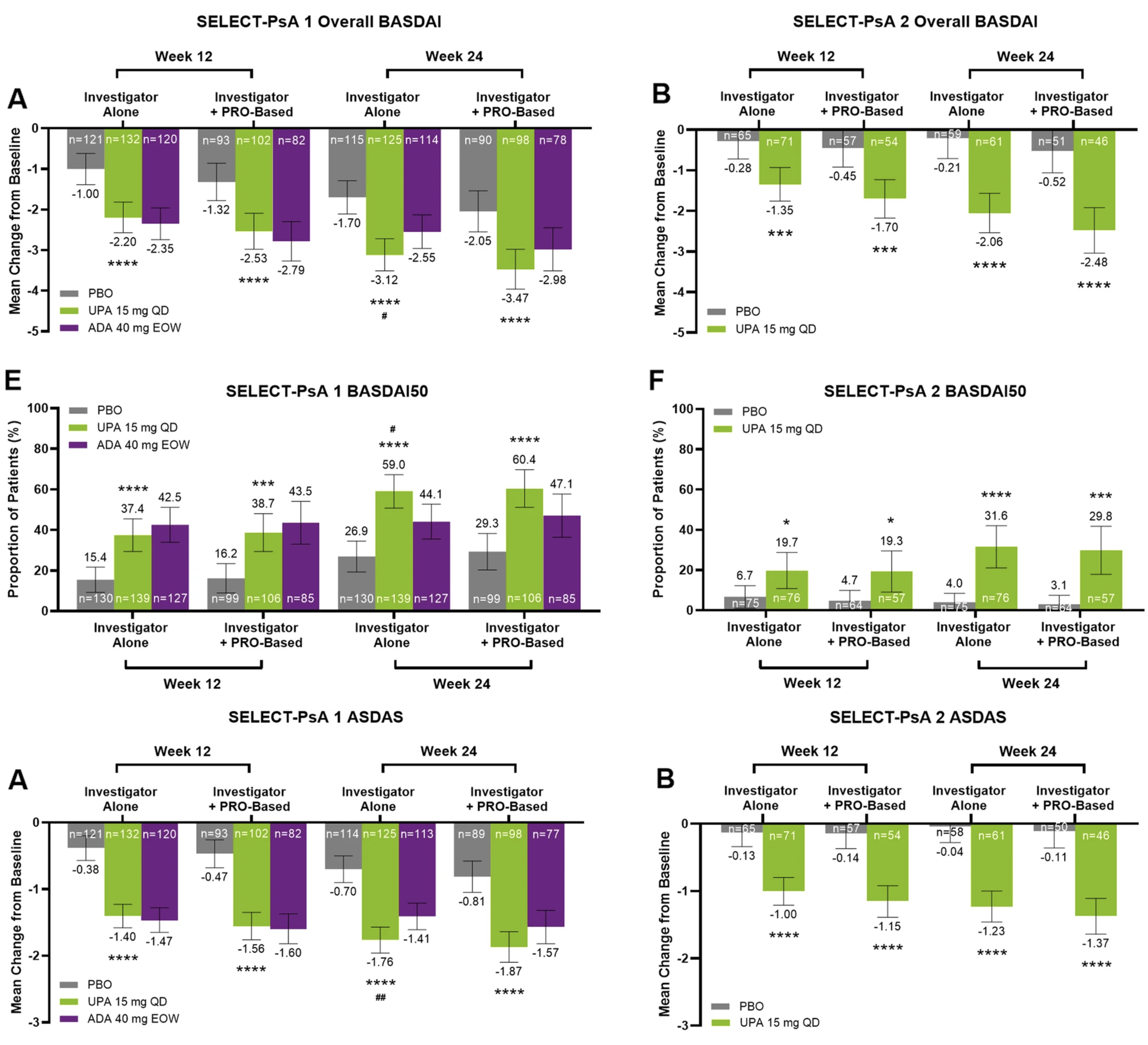
A number of post hoc analyses of studies in PsA have been done to determine if axial disease also improves with the intervention. These analyses are hampered by a lack of validated case definition and non-specific outcome measures. The MAXIMISE study is the only study that attempted to specifically study axial PsA using MRI. However, the case definition was not validated and only around 60% of the patients had MRI changes (Baraliakos X, et al). Studies on axial PsA will be facilitated by MRI-based enrolment and response criteria in conjunction with clinical measures.
This post-hoc analysis convincingly demonstrates that upadacitinib has beneficial effects on axial symptoms in patients with axPsA. But does that mean a beneficial impact has been demonstrated in alleviating axial inflammation or are the observed effects a by-stander phenomenon in patients whose arthritis has improved to such a degree that patients are self-reporting improvements in a global manner in the non-specific BASDAI and ASDAS scores? There is no accepted case definition for axPsA but this will likely require imaging features, especially on MRI of the sacroiliac joints and/or spine. Clinical assessment of axial disease can be confused with degenerative spinal disorders because features of inflammatory back pain lack sensitivity and specificity for axial inflammation. Degenerative disorders are more frequent in the older demographic of PsA patients who develop axial inflammation. A more convincing case for the efficacy of treatment for axPsA can be made in trials selecting patients according to the presence of imaging features on MRI, especially a degree of inflammation in the sacroiliac joints and/or spine greater than would be expected in degenerative disorders. Moreover, it is essential to demonstrate improvement in the most objective parameter of axial inflammation, namely, bone marrow edema on MRI in the sacroiliac joints and/or spine.