


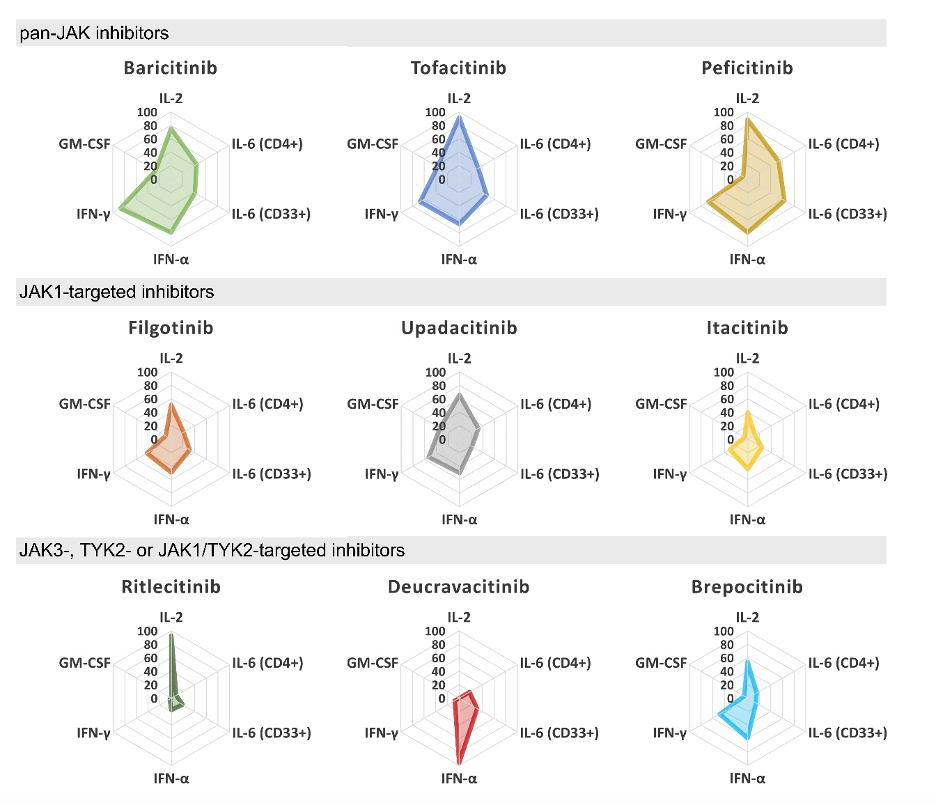
JAK inhibitors are mysterious drugs. Despite the fact that they are designed to target a well-known enzymatic activity, we still don’t know why they work in axial spondyloarthritis or how to explain some of their side effects. The study by Virtanen et al. shows that the JAK inhibitors currently approved for rheumatic disease indications performed largely similar when tested in vitro for their ability to block cytokine receptor signaling in immune cells. Most JAK inhibitors work by binding non-covalently to the ATP binding site of the kinase domain. Ritlecitinib and Deucravacitinib have unique JAK targeting mechanisms giving them significantly higher selectivity for JAK3 and TYK2, respectively, and distinct cytokine receptor inhibition profiles in vitro. Whether a higher selectivity for individual JAKs results in better efficacy or reduced side effects in vivo remains to be shown.
This study was designed to provide a comprehensive in vitro analysis on direct JAK-related effects (JAK selectivity, cytokine inhibition profile in heathy and in patient cells) and comparison of JAKi evaluated for the treatment of rheumatic diseases. Inhibition of JAK kinase activity did not directly translate into cellular inhibition of JAK-STAT signaling. Despite differences in JAK-selectivity, the cytokine inhibition profiles of currently approved JAKi were highly similar with preference for targeting inhibition of JAK1-mediated cytokines. This basic work might explain why clinical efficacy data for tofacitinib and upadacitinib appears similar in phase III trials of bioDMARD-naïve radiographic axSpA. But does not shed light on the mechanism of action of JAKi in axSpA since we know from prior trials in AS that targeting IL6 is not beneficial.