


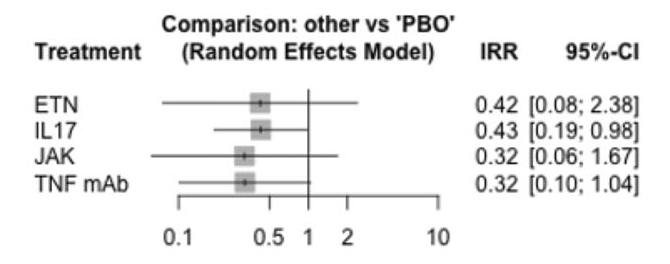
This interesting study assessed whether TNF, IL17, and JAK inhibitors lowered the risk of AAU. There is strong evidence that TNF inhibitors are effective in treating and preventing flares of uveitis. Previous studies of IL17A inhibitors in non-infecious AAU did not show benefit. More recent data presented at ACR showed a lower incidence of AAU in patients treated with bimekizumab and secondary analysis of upadacitinib also showed reduced AAU events. A previous NMA published by Roche et al showed a reduction of AAU events with TNF but not with IL17. This study showed TNF, IL17, and JAK inhibitors all reduced rates of AAU. The different result of this study may have been due to combining IL17A and A/F data. We have not seen data on AAU events from secukinumab and ixekizumab trials in AxSpA or PsA.
A valid NMA should satisfy the assumption of transitivity i.e. that there are no systematic differences between the studies other than the treatments being compared. But only 28 studies reported on history of AAU at baseline and where this was reported the proportion of patients with a history of AAU ranged from 3% to 46% between RCTs, with some imbalance between the treatment and placebo groups. A prior history of AAU is a major risk factor for de novo AAU events. In one study the incidence of AAU flares on one TNFi mAb, adalimumab, was 14 per 100 patient-years, with 43% having a prior history of AAU (van Denderen et al.). In the RHAPSODY study, the incidence of AAU flares on adalimumab was 7.4 per 100 patient-years, with 22% having a history of AAU (Rudwaleit et al.). Combining data from bimekizumab RCTs, this agent inhibiting both IL17A and -F, with data from other IL17i RCTs may not be appropriate in view of recent data suggesting benefit for AAU in axSpA (Rudwaleit et al.), while studies of IL17i in non-infectious AAU RCTs did not show benefit (Dick et al.).